





Research
Current Projects:
Rab GTPases play a multitude of roles in vesicular transport, including regulation of vesicle formation, vesicle motility, vesicle tethering, membrane fusion, and membrane remodeling. Rab GDP dissociation inhibitor (GDI) is a protein that binds to Rabs, keeping Rab in the GDP-bound state, as well as delivering and placing Rabs in the correct membrane compartment. The mechanism for releasing Rab from GDI and delivering the Rab to the correct membrane is unknown and is likely to involve proteins that bind and interact with GDI.
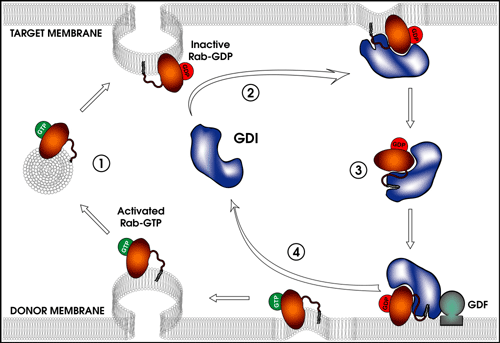
Function and cycling of Rab GDP dissociation inhibitor. Figure by Ryan Takeshita ’04.
This research program has previously cloned the Drosophila GDI gene, determined the Drosophila GDI crystal structure, isolated and characterized Drosophila GDI mutations, and located the GDI mutations within the GDI crystal structure (Ricard et al., 2001). Prior work has discovered 14 genomic regions that interact genetically with a GDI null allele, creating a female sterile phenotype. Current projects in the lab are identifying the interacting gene(s) in these regions, determining the nature of the female sterility in the interaction, using a GST pull-down system to identify proteins that interact with GDI, and also using a yeast two-hybrid system to isolate GDI-interacting proteins.
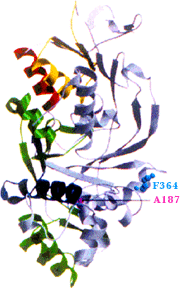
GDI structure (Ricard et al. 2001)
Selected Publications:
Ricard, C.S., J.M. Jakubowski, J.W. Verbsky, M.A. Barbieri, W.M. Lewis*, G.E. Fernandez, M. Vogel, C. Tsou*, V. Prasad*, P. Stahl, G. Waksman and C.M. Cheney. 2001. Drosophila rab GDI mutants show developmental defects but normal rab membrane extraction. Genesis 31: 17-29. [abstract]
Garrett, M., J.E. Zahner, C.M. Cheney, and P. Novick. 1994. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 13: 1718-1728. [abstract]
Cheney, C.M., N.G. Kravit, and J.W. Verbsky. 1993. A new myosin I gene in Drosophila. Biochem. Biophys. Res. Comm. 3: 1280-1288. [abstract | article]
Garrett, M.D., A.K. Kabcenell, J.E. Zahner, T. Sasaki, Y. Takai, C.M. Cheney and P.J. Novick. 1993. Interaction of Sec4 with GDI proteins from bovine brain, Drosophila melanogaster and Saccharomyces cerevisiae: Conservation of GDI membrane dissociation activity. FEBS Letters 331: 233-238. [abstract]
Zahner, J.E., and C.M. Cheney. 1993. A Drosophila homolog of bovine smg p25a GDP dissociation inhibitor (GDI) undergoes a shift in isoelectric point in the developmental mutant, quartet. Molec. Cell. Biol. 13: 217-227. [abstract | article]
Zahner, J.E., and C.M. Cheney. 1990. quartet: a Drosophila developmental mutation affecting chromosome separation in mitosis. Devel. Gen. 11: 27-40. [abstract]