Pyruvate kinase
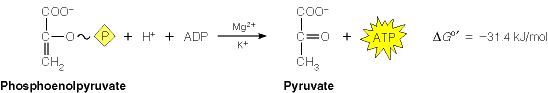
Pyruvate kinase (PK) catalyzes the final reaction of glycolysis, the conversion of phosphoenol pyruvate to pyruvate, which is coupled to the formation of ATP. This reaction is irreversible in cells.
from Matthews, Van Holde, and
Ahern; Biochemistry, 3rd
edition
|
Activator |
Inhibitors |
|
Fructose-1,6-bisphosphate |
ATP Alanine Acetyl-CoA |
Hormonal Control of
PK
PK in the liver is sensitive to carbohydrate ingestion. Glucagon causes the phosphorylation of PK, which renders it much less active. Insulin diminishes this effect, and increases the concentration of the dephosphorylated, more active enzyme.
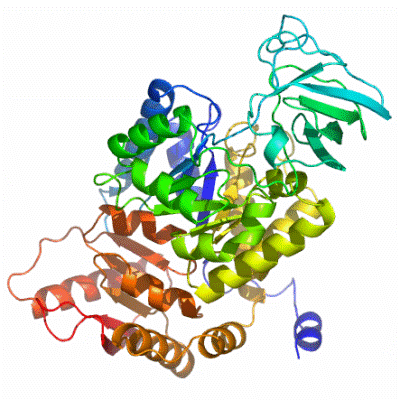
Structure of PK
|
http://sgc.utoronto.ca/SGC-WebPages/StructureDescription/1ZJH.php |
PK is made of 4 identical subunits. To the left is the structure of PKM2, an isomer of PK found in human muscle. A different isomer, pictured below, is found in the liver and erythrocytes. |
|
University of Pavia,
Protein Crystallography Group http://www.unipv.it/biocry/structures.php
has an interactive look at its structure |