Hippocampal Research:
Changes with Aging in Cyclic AMP-Dependent Synaptic Transmission and Synaptic Plasticity in Rat Hippocampus
Research in our laboratory is focused upon a brain region that is critical for transferring information from short-term storage into long-term memory, the hippocampus. Memory disorders that accompany a wide variety of neurological diseases, including Alzheimer’s disease, as well as memory deficits associated with normal aging, may be a direct consequence of hippocampal dysfunction. Neurons in the hippocampus, as in all other brain areas, communicate with each other via chemical signals at special junctions known as synapses. When an electrical signal traveling through a neuron reaches the end of the cell, the electrical signal causes the chemical messenger, or neurotransmitter, to be released into the synapse, where it is then detected by adjacent neurons via receptor proteins. These postsynaptic receptors then convert the chemical signal back into an electrical signal. Most neuroscientists believe that memories are stored via alterations in the strength of chemical synapses. A fascinating property of synapses in the hippocampus is that when they are used repetitively, the signal transmitted from one neuron to the next is strengthened somehow — either via an increase in the amount of neurotransmitter that is released from the neuron, or via an increase in the sensitivity of the postsynaptic neuron to the neurotransmitter. This increase in synaptic efficacy is more or less permanent, and is therefore referred to as “long-term potentiation”, or LTP. Our central goals in the lab are to elucidate some of the molecular mechanisms underlying LTP, and to reach a better understanding of how such mechanisms change with aging.
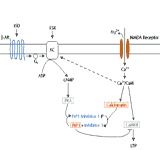
Nerve cells and cells in other organs respond to many chemical neurotransmitters by binding the transmitter via cell surface receptor proteins; this interaction sets up a domino-like cascade of intracellular chemical events, eventually leading to the production of small signaling molecules referred to as “second messengers”. A common second messenger is cyclic adenosine monophosphate (cyclic AMP), which is produced from ATP by a membrane enzyme, adenylate cyclase. The largest neurons of the hippocampus respond to the transmitter norepinephrine by producing cyclic AMP, which leads to excitation of the cells. My previous work has shown that hippocampal neurons of aged rats fail to respond properly to norepinephrine. Moreover, a common theme emerged in studies of other brain regions: transmitters that modulate neuronal activity via the production of cyclic AMP do not appear to affect aged neurons as they would young neurons. The immediate question that this research will address is: what step in the cellular response to norepinephrine is altered in aged rats? Is there a problem at the receptor level? Or an alteration in adenylate cyclase activity? Or is there an inability of cyclic AMP to activate its targets? All of these possibilities will be investigated.
There are several forms of synaptic potentiation that require intact cyclic AMP pathways. These forms of synaptic potentiation may be disrupted in the aged animals. An intriguing aspect of this work is the possibility that a molecular defect in aged hippocampal neurons affects the way that these neurons interact with one another. As a consequence, the ability of an aged animal to learn and retain new information would be impaired. By testing the performance of aged vs. young rats on certain learning tasks before and after manipulating this critical cyclic AMP cascade, we will in fact be able to correlate changes at the molecular level to changes at the behavioral level. In the past, such correlations have been difficult to do in mammalian systems. Recent advances in molecular biology, physiological recording techniques, and assessment of memory impairment in rodents, however, have made these studies possible. Thus, this is a very exciting era in which to study the neurobiology of aging.
The elucidation of age-related changes in hippocampal function is critical to understanding the gradual (but not necessarily inevitable) memory losses that are associated with normal aging, as well as the more severe changes in memory associated with pathological conditions like Alzheimer’s Disease. The brain region most affected by Alzheimer’s Disease is the hippocampus. Moreover, patients with strokes that are localized to the hippocampus display an inability to convert information from short-term into long-term storage. Clearly, a better understanding of this critical brain region may enhance our ability to learn and retain new information in our later years.
In addition, the findings of this project with regard to changes in the cyclic AMP second messenger cascade may be generalized to other organ systems of the body. A wide variety of pharmacological agents, from antihypertensive drugs to antidepressants, rely upon intact cyclic AMP pathways for their mechanism of action. These agents are prescribed more frequently in elderly patients, often in combination with other drugs. Lack of effectiveness of pharmaceutical agents in elderly patients may eventually be attributed to defects in particular second messenger pathways, such as the cyclic AMP pathway, rather than simple changes in the way that elderly people metabolize the drugs. Hence, these studies may influence pharmaceutical treatments of disease in the elderly. In particular, several nootropic drugs (drugs to treat memory loss) that are currently under development act by enhancing cyclic AMP levels. It is possible that these drugs might be less efficacious in the elderly. The goals of this project are consistent with what the goals of aging research should be — not simply lengthening life, but enhancing and preserving its quality throughout the later years.